HIV
Maturation Inhibitor Bevirimat Demonstrates Promising Safety and Efficacy in Patients
with Responsive HIV Genotype
 | The
investigational HIV maturation inhibitor bevirimat (MPC-4326, formerly PA-456)
was well-tolerated in a small study and showed good antiviral activity against
HIV with specific Gag protein variations, researchers reported at the 49th Interscience
Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2009) last week in
San Francisco. |
|
By
Liz Highleyman Maturation
inhibitors interfere with the final stage of HIV replication, when viral proteins
are assembled, packaged, and "bud" out through the host cell membrane
to form new virus particles. Bevirimat
targets HIV Gag proteins that make up the capsid, or inner "capsule"
that contains the viral genetic material. 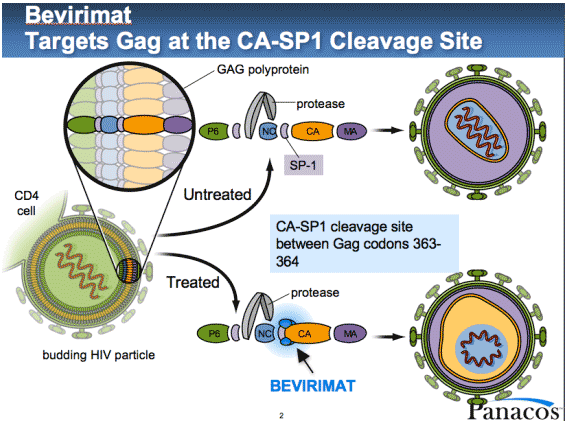
Bevirimat
is the most advanced maturation inhibitor candidate in development. Originally
known by the Panacos Pharmaceuticals designation PA-457, the drug demonstrated
promising activity in early studies, but a tablet formulation had poor bioavailability
and produced
a lower-than-expected response. Researchers
then conducted further studies using a more bioavailable liquid formulation, with
improved outcomes, as
reported at ICAAC 2008. Post-hoc analyses showed that patients whose HIV carried
specific polymorphisms (variations at a single site on the genome) were more likely
to respond to bevirimat. Myriad Pharmaceuticals acquired the drug in January 2009
and renamed it MPC-4326. This
year, researchers presented findings from a Phase 2 trial known as Study 204.
This trial included 32 participants, most of whom were starting antiretroviral
therapy (ART) for the first time. However, 6 were treatment-experienced patients
with resistance to approved drugs; these individuals underwent a "washout"
period of at least 3 days before starting bevirimat. All
participants were men, the average age was 40 years, most (97%) were white (the
study was conducted in Australia), the mean CD4 count was about 400 cells/mm3,
and the mean viral load was about 63,000 copies/mL. In
this open-label study, patients received bevirimat monotherapy at doses of 200
mg or 300 mg twice-daily (BID) with food for 14 days, using a 50 mg tablet shown
to be better absorbed than the original tablet formulation. Investigators
looked at response rates stratified by predicted response, using a predictive
algorithm based on 5 polymorphisms in the HIV Gag gene that was developed using
data from 100 participants in previous trials. The algorithm identified responders
with 80% accuracy and non-responders with 89% accuracy. Results  | Patients
achieved trough (lowest between doses) drug levels exceeding the 90% effective
concentration (EC90) using both the 200 mg and 300 mg dosages. |  | After
14 days, viral load decreased by an average of 0.54 log10 copies/mL in the 200
mg arm and by 0.70 log10 copies/mL in the 300 mg arm. |  | Participants
classified as likely responders according to the algorithm had an average viral
load decrease of 1.15 log10 copies/mL, compared with 0.17 log10 copies/mL for
predicted non-responders. |  | Overall,
viral load decreases did not differ significantly between the 200 mg and 300 mg
arms. |  | Among
predicted responders, however, mean HIV RNA decline was significantly greater
in the 300 mg compared with the 200 mg arm (1.38 vs 0.89 log10 copies/mL, respectively). |  | The
drug was generally well-tolerated, with most adverse events being mild to moderate.
|  | The
most common adverse events were headache (22%) and gastrointestinal symptoms including
nausea (16%), diarrhea (16%), and constipation (13%). |  | There
were no serious clinical adverse events or laboratory abnormalities. |
The
researchers concluded that the bevirimat 50 mg tablet formulation "was safe
and well tolerated at 200 and 300 BID."Holdsworth
House Med. Practice, Sydney, Australia; Taylor Square Private Clinic, Sydney,
Australia; Myriad Pharmaceuticals, Salt Lake City, UT; AIDS Research Iniitative,
Sydney, Australia; St Vincents Hosp, Sydney, Australia. New
100 mg Tablet Another
research team presented a poster describing findings from a pharmacokinetic study
of 35 patients testing a new 100 mg tablet formulation of bevirimat. Over
15 days of dosing, the drug demonstrated good bioavailability. Twice-daily dosing
produced high minimum concentrations compared with once-daily dosing. Food had
a minimal effect on overall exposure, though it delayed reaching the maximum concentration.
Here, too, the most common adverse events were headaches and gastrointestinal
symptoms, including diarrhea (about 30%), nausea, and abdominal cramps. The
company indicated that it plans to initiate a phase 2b "in the near future"
using the 100 mg tablet formulation. Quest
Clinical Research, San Francisco, CA; Private Practice, Ft. Lauderdale, FL; AIDS
Research Consortium, Atlanta, GA; CRINE, Boston, MA; Myriad Pharmaceuticals, Inc.,
Salt Lake City, UT. 9/25/09 References M
Bloch, N Bodsworth, M Fidler, and others. Efficacy, safety and pharmacokinetics
of MPC-4326 (bevirimat dimeglumine) 200mg BID and 300mg BID monotherapy administered
for 14 days in subjects with HIV-1 infection. 49th Interscience Conference on
Antimicrobial Agents and Chemotherapy (ICAAC 2009). San Francisco. September 12-15,
2009. Abstract H-1230. J
Lalezari, G Richmond, M Thompson. Pharmacokinetics and safety of a novel 100 mg
tablet formulation of MPC-4326 in subjects with HIV-1 infection. ICAAC 2009. Abstract
A1-1309. Other
source
Myriad Pharmaceuticals. Myriad Pharmaceuticals Announces Two Abstracts
Selected for Presentation At 2009. Press release. September 10, 2009.
|